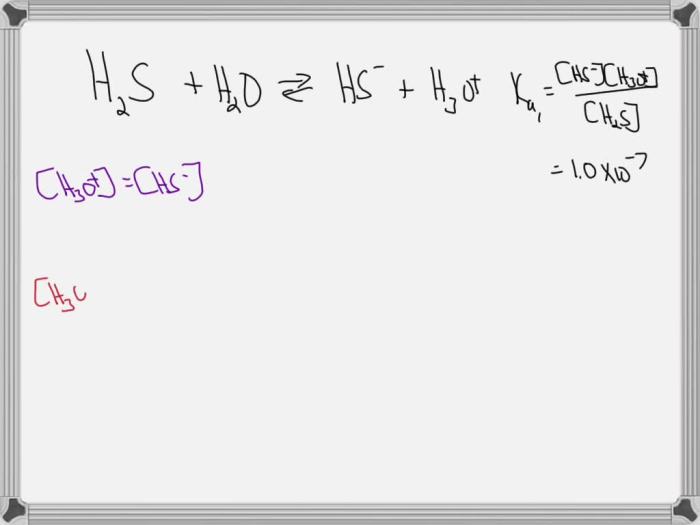To a first approximation the ionization constant of h2s is – To a first approximation, the ionization constant of H2S, denoted as Ka, provides a quantitative measure of its ability to dissociate in aqueous solutions, releasing H+ ions. Understanding Ka is crucial for comprehending the acidic or basic nature of H2S and its behavior in various chemical and biological systems.
This article explores the concept of ionization constant, its significance in understanding the acidity of H2S, and the factors that influence its value. We will also delve into the applications of Ka in chemistry, biology, and environmental science, highlighting its importance in predicting the behavior of chemical systems.
Ionization Constant of H2S
The ionization constant, denoted as K a, is a quantitative measure of the acidity or basicity of a compound in a solution. It represents the equilibrium constant for the dissociation of an acid into its conjugate base and a hydrogen ion (H +).
The formula for calculating the ionization constant is:
Ka= [H +][A –]/[HA]
where:
- [H +] is the molar concentration of hydrogen ions
- [A –] is the molar concentration of the conjugate base
- [HA] is the molar concentration of the acid
The ionization constant provides insights into the strength of an acid. A higher K avalue indicates a stronger acid, as it dissociates more readily into ions. Conversely, a lower K avalue indicates a weaker acid.
First Approximation of Ionization Constant
The first approximation of the ionization constant, denoted as K a1, is obtained by assuming that the concentration of the conjugate base is negligible compared to the concentration of the acid.
This assumption is valid when the acid is weak and only a small fraction of it dissociates. Under these conditions, the following approximation can be made:
Ka1= [H +]/[HA]
The first approximation provides a simplified estimate of the ionization constant, which can be useful for weak acids.
However, it’s important to note that this approximation may not be accurate for strong acids, where the concentration of the conjugate base is significant.
Factors Affecting Ionization Constant of H2S
The ionization constant of H2S is influenced by several factors:
Temperature
The ionization constant generally increases with increasing temperature. This is because higher temperatures promote the dissociation of acids into ions.
Solvent
The solvent used can also affect the ionization constant. Solvents with high dielectric constants, such as water, favor the dissociation of acids, leading to higher K avalues.
Ionic Strength
The presence of other ions in the solution can decrease the ionization constant of H2S. This effect, known as the common ion effect, occurs because the added ions compete with the hydrogen ions for the conjugate base, reducing the concentration of free hydrogen ions.
Applications of Ionization Constant, To a first approximation the ionization constant of h2s is
The ionization constant has numerous applications in various fields:
Chemistry
In chemistry, the ionization constant is used to determine the strength of acids and bases, predict the pH of solutions, and design buffer systems.
Biology
In biology, the ionization constant plays a crucial role in understanding enzyme activity, protein folding, and the regulation of cellular processes.
Environmental Science
In environmental science, the ionization constant is used to assess the acidity of water bodies, predict the fate of pollutants, and design wastewater treatment systems.
General Inquiries: To A First Approximation The Ionization Constant Of H2s Is
What is the ionization constant?
The ionization constant, denoted as Ka, is a quantitative measure of the extent to which an acid dissociates in water, releasing H+ ions.
How is the ionization constant of H2S determined?
The ionization constant of H2S can be determined experimentally by measuring the pH of a solution containing H2S and using the Henderson-Hasselbalch equation.
What factors affect the ionization constant of H2S?
The ionization constant of H2S is influenced by several factors, including temperature, solvent, and ionic strength.
What are the applications of the ionization constant?
The ionization constant has applications in various fields, such as chemistry, biology, and environmental science, where it is used to predict the behavior of chemical systems and understand the interactions of acids and bases.