The molar mass of naoh is 40.00 g mol – The molar mass of NaOH, standing at 40.00 g/mol, is a fundamental property that governs its behavior in chemical reactions. This article delves into the significance, applications, and practical implications of this crucial molecular characteristic, providing a comprehensive understanding of its role in chemistry and beyond.
Beyond its theoretical importance, the molar mass of NaOH finds practical applications in various fields. From its use in stoichiometric calculations to its significance in industrial processes, this property plays a vital role in shaping the chemical landscape.
Molar Mass of NaOH: A Comprehensive Guide
The molar mass of sodium hydroxide (NaOH) is a fundamental property that plays a crucial role in various chemical and industrial applications. This article delves into the concept of molar mass, explores the significance of NaOH’s molar mass (40.00 g/mol), and discusses its practical applications and safety considerations.
1. Molar Mass Concept

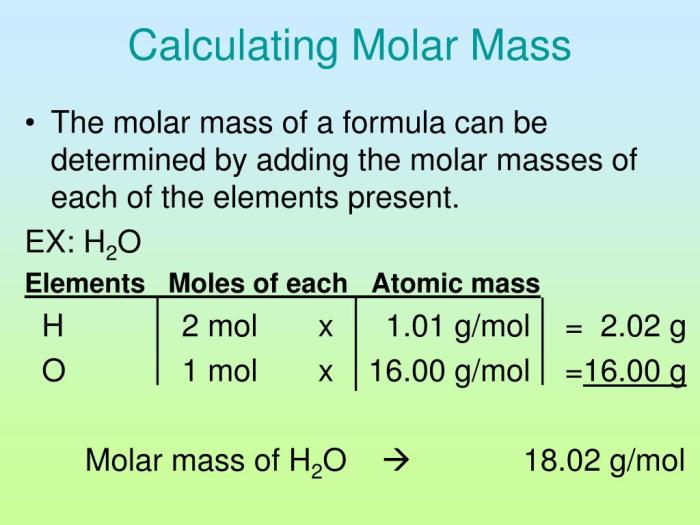
Molar mass is the mass of one mole of a substance. It is a measure of the average mass of all the atoms or molecules in a substance. The molar mass of an element is equal to its atomic mass, while the molar mass of a compound is equal to the sum of the atomic masses of all the atoms in its formula.
For example, the molar mass of sodium (Na) is 22.99 g/mol, the molar mass of oxygen (O) is 16.00 g/mol, and the molar mass of hydrogen (H) is 1.01 g/mol. Therefore, the molar mass of NaOH is 22.99 g/mol + 16.00 g/mol + 1.01 g/mol = 40.00 g/mol.
2. Molar Mass of NaOH
The molar mass of NaOH (40.00 g/mol) is a significant property that determines its behavior and reactivity in chemical reactions. It indicates that one mole of NaOH contains 40.00 grams of the compound.
NaOH is a strong base that readily dissociates in water to form sodium ions (Na+) and hydroxide ions (OH-). Its high molar mass contributes to its ability to neutralize acids and form salts.
3. Applications of NaOH Molar Mass
The molar mass of NaOH is used extensively in chemistry and industry:
- Stoichiometric calculations:Molar mass is essential for determining the quantities of reactants and products involved in chemical reactions.
- Titrations:NaOH is commonly used in titrations to determine the concentration of acids. Its molar mass allows for accurate determination of the number of moles of NaOH used.
4. Conversions and Calculations
| Quantity | Conversion |
|---|---|
| Grams to Moles | Grams ÷ Molar Mass (40.00 g/mol) |
| Moles to Grams | Moles × Molar Mass (40.00 g/mol) |
| Grams to Molecules | Grams ÷ Molar Mass (40.00 g/mol) × Avogadro’s Number (6.022 × 1023 molecules/mol) |
| Molecules to Grams | Molecules ÷ Avogadro’s Number (6.022 × 1023 molecules/mol) × Molar Mass (40.00 g/mol) |
Flowchart for Calculating Molar Mass of NaOH:
- Determine the atomic masses of sodium (Na), oxygen (O), and hydrogen (H) from the periodic table.
- Multiply the atomic mass of each element by the number of atoms of that element in the formula of NaOH (1 Na, 1 O, 1 H).
- Add the masses of all the atoms to obtain the molar mass of NaOH.
5. Related Compounds
The molar masses of related compounds, such as NaOH, KOH, and LiOH, are closely related:
- NaOH: 40.00 g/mol
- KOH: 56.11 g/mol
- LiOH: 23.95 g/mol
These compounds have similar properties as they are all strong bases. However, their different molar masses result in variations in their reactivity and applications.
6. Practical Applications: The Molar Mass Of Naoh Is 40.00 G Mol
The molar mass of NaOH is used in various practical applications:
- Titrations:NaOH is a standard solution used in titrations to determine the concentration of acids.
- Neutralization reactions:NaOH is used to neutralize acids, producing salts and water.
- Soap and detergent production:NaOH is used in the production of soaps and detergents by reacting with fats and oils.
7. Safety Considerations
NaOH is a corrosive substance and should be handled with care. It can cause skin burns and eye damage. When working with NaOH, it is essential to wear appropriate protective gear, such as gloves, goggles, and a lab coat.
NaOH should be stored in a cool, dry place away from incompatible materials, such as acids and metals. It should be disposed of properly according to local regulations.
Question Bank
What is the molar mass of NaOH?
The molar mass of NaOH is 40.00 g/mol.
How is the molar mass of NaOH used in chemistry?
The molar mass of NaOH is used in stoichiometric calculations to determine the amount of reactants and products involved in chemical reactions.
What are the practical applications of the molar mass of NaOH?
The molar mass of NaOH is used in various practical applications, including titrations, neutralization reactions, and the production of soap and detergents.